Fertomid
General Information about Fertomid
The dosage of Fertomid varies relying on the person's situation, but it is typically taken orally for 5 days of the menstrual cycle. It is essential to comply with the prescribed dosage and instructions to ensure maximum effectiveness and avoid any potential unwanted side effects. Some common unwanted aspect effects of Fertomid include sizzling flashes, temper swings, complications, and breast tenderness. These side effects are usually delicate and go away on their very own.
In conclusion, Fertomid is a popular fertility agent that has helped many ladies successfully conceive and start a family. It is a relatively protected and reasonably priced treatment that stimulates ovulation in ladies with ovulation problems. However, it's critical to grasp the potential risks and considerations associated with Fertomid and to consult with a healthcare skilled for an individualized remedy plan. With the proper guidance and help, fertility therapies like Fertomid can convey a glimmer of hope for couples who dream of turning into dad and mom.
Fertomid, also called clomiphene citrate, is a nonsteroidal fertility treatment that belongs to a class of drugs referred to as selective estrogen receptor modulators (SERMs). It works by blocking estrogen receptors within the mind, which then alerts the body to provide more follicle-stimulating hormone (FSH) and luteinizing hormone (LH). These hormones are essential in the process of ovulation, where the ovaries release an egg each month.
One of the numerous benefits of Fertomid is that it's relatively reasonably priced in comparability with other fertility treatments. It also has a excessive success fee, with research exhibiting that about 70% of ladies who take Fertomid will ovulate, and around 35% will turn out to be pregnant within six cycles of use. However, it is essential to note that the success of fertility therapies varies from person to person and is dependent upon many components, together with age, general well being, and underlying causes of infertility.
Like any medicine, Fertomid does come with some potential risks and considerations. Women with a history of liver disease, ovarian cysts, or uterine fibroids ought to inform their doctor before starting Fertomid. Additionally, Fertomid could increase the risk of multiple pregnancies (twins/triplets), which may result in potential issues during being pregnant and supply.
Fertomid is commonly prescribed to ladies who're experiencing ovulation disorders, which is one of the most common causes of infertility. This could be because of situations like polycystic ovary syndrome (PCOS) or premature ovarian failure. Fertomid can also be used for women who've irregular menstrual cycles or those who are undergoing fertility remedies like in vitro fertilization (IVF).
In some cases, Fertomid may not be the simplest option for treating infertility. If a lady has blocked fallopian tubes or if her male companion has fertility issues, different therapies like IVF may be really helpful as an alternative. It is always important to seek the advice of with a fertility specialist to determine the most effective course of action for each particular person's unique situation.
Fertility has always been a subject of great importance and curiosity, particularly for couples who are making an attempt to begin a household. For some, fertility could come naturally, however for others, it could require slightly additional assist. This is the place medicines like Fertomid come into play. Fertomid is a fertility agent that is used to stimulate ovulation in ladies who are having difficulty getting pregnant.
In the degradation of haemoglobin pregnancy updates purchase fertomid now, the molecule is broken down to two subunits, which are bound to haptoglobin, the complex being rapidly internalized in the hepatocyte after binding to the haptoglobin complex receptor. In the presence of haemolysis, serum haptoglobin levels are greatly reduced or absent. However, haptoglobin is an acute-phase protein and levels will increase in the presence of inflammation. Chronic haemolytic anaemia may increase the iron content of the body through increased iron absorption as a result of anaemia coupled to the retention of the haem iron following binding to haptoglobin and haemopexin. In rare cases of inherited haemolytic anaemia, this iron overload may be sufficient to produce clinically important effects, particularly if there is coinheritance of a haemochromatosis gene. In most haemolytic anaemias, owing to membrane defects, the destruction of red cells takes place extravascularly in the reticuloendothelial system, particularly in the spleen, and the iron is retained. When destruction is intravascular, free haemoglobin will be released into the plasma, producing haemoglobinaemia and methaemalbuminaemia, and will pass through the glomerulus to produce haemoglobinuria and haemosiderinuria. Increased red cell production leads to expansion of the red cell precursor compartment of the bone marrow, as described above. There are also changes in the structure of the marrow as a consequence of the chronic anaemia, which allows the early release of reticulocytes and, in more marked cases of haemolytic anaemia, nucleated red cells and even myelocytes. In the peripheral blood, the polychromasia and macrocytosis of reticulocytosis are the result of this increased throughput and release. The increased cell production requires an increased supply of folate which, at least theoretically, can produce folate deficiency unless supplements are given. It is usual to give folic acid (400 g daily or 5 mg once weekly) to people with chronic haemolytic anaemia. Increased red cell destruction Unconjugated hyperbilirubinaemia Mild jaundice Increased risk of gallstones Increased urinary and faecal urobilinogen Decreased serum haptoglobin and haemopexin Extravascular changes Increased iron stores Splenomegaly Intravascular changes Haemoglobinaemia and haemoglobinuria Haemosiderinuria Methaemalbuminaemia Decreased iron stores Increased red cell production Marrow expansion: bone changes Increased erythropoiesis: myeloid/erythroid ratio Reticulocytosis: polychromasia Increased folate requirements: macrocytosis by appropriate metabolic machinery and structural organization of the proteins. Accordingly, inherited haemolytic disorders can be classified into three major groups: (i) genetic disorders of haemoglobin (see Chapter 6); (ii) abnormal membrane (including the cytoskeleton) and (iii) abnormal metabolism (enzymopathies). Classification Because of the unique structural and functional specialization of the mature red cell, the impact on it of a wide range of exogenous or endogenous changes is relatively uniform: the cell will be destroyed prematurely. According to the site of the primary change, haemolytic disorders have been traditionally classified as being due either to intracorpuscular or to extracorpuscular causes. According to the nature of the primary change, haemolytic disorders have also been classified as inherited or acquired. These two classifications correlate almost completely with each other, in that extracorpuscular causes are usually acquired, whereas intracorpuscular causes are usually inherited. One notable exception is paroxysmal nocturnal haemoglobinuria, a disease in which an intracorpuscular defect is acquired as a result of a somatic mutation (see Chapter 9). Although in every cell all molecules and organelles are naturally interdependent, it is convenient to consider the red cell as a conveyance for a large amount of haemoglobin contained in a plasma membrane, the stability of which is maintained Red cell membrane disorders the red cell membrane the red cell membrane, like all other cell membranes, consists of a lipid bilayer that is stabilized and given specific properties by the proteins, glycolipids and other specialized molecules and structures with which it is associated. The lipid bilayer consists of approximately equal molar quantities of phospholipids and cholesterol molecules. The charged phosphatidyl groups of the phospholipids are hydrophilic and form the outer and inner surfaces of the bilayer. Maintenance of the asymmetry and the proper function of the membrane requires energy. The acyl chains of the diacylphosphatidylglycerides are hydrophobic non-polar domains and they form hydrophobic bonds with the acyl groups of the opposite layer. Cholesterol is present in roughly equimolar amounts and determines the fluidity of the membrane. The normal biconcave shape and function of the red cell membrane are determined by the membrane proteins and their interactions with the lipid bilayer and with each other. The integral proteins have strong hydrophobic domains that associate with the hydrophobic part of the bilayer. Many of these integral proteins span the membrane and provide channels between the plasma and cytosolic compartments. The cytosolic inner domains of these proteins interact with each other and with the second main group, the proteins of the membrane skeleton. Genetic abnormalities that produce spherocytosis mainly have mutations affecting the vertical connections. Mutations of the horizontal system usually produce elliptocytosis or more bizarre-shaped changes. In addition to the compartments mentioned so far, there are numerous surface proteins that provide the main interface with the plasma, including the blood group systems and other receptors. Many of these molecules are heavily glycosylated, as are the integral proteins, the glycophorins; sialic acid, which comprises the main side-chain of the glycophorins, contributes the most to the negative surface change of the erythrocyte. The integral proteins and vertical interaction the two major integral proteins that span the lipid bilayer are band 3 (the anion channel protein) and glycophorin C. The main protein of the membrane skeleton is spectrin, consisting of two subunits, and, which associate side by side to produce a heterodimer. The tail end of the dimer makes contact with a short actin filament composed of 14 monomers; the interaction between spectrin and actin is stabilized by protein 4. Binding of spectrin dimers to actin filaments produces the more or less hexagonal network of spectrin tetramers on the inner surface of the membrane associated with the lipid bilayer. Spectrin qualitative defects that affect these horizontal interactions tend to induce a loss of structural stability of the membrane and elliptocytosis.
Therefore women's health center university of maryland 50 mg fertomid buy free shipping, it is highly recommended to separate IgM and IgG fractions before testing to decrease the incidence of both false-positive and false-negative IgM test results. Rapid and simple methods for the removal of interfering rheumatoid factor and IgG molecules from serum have been developed. These include selective absorption of IgM to a solid phase and removal of IgG by using hyperimmune antihuman IgG antibody, staphylococcal protein A, or recombinant protein G from group G streptococci. More recently, reverse capture solid-phase IgM assays have been used as an alternative approach to avoiding false-positive or falsenegative results. Because IgM does not cross the placenta, a positive result from a single serum specimen from an infected newborn is diagnostic. This complicates the interpretation of test results, particularly for pregnant women or immunocompromised patients, and may not be predictive of recent or active infection. Like newborns, immunocompromised individuals also may have a delay in IgM production or may be unable to mount a significant IgM antibody response. Ganciclovir-resistant clinical isolates have also been recovered from bone marrow and solid organ transplant recipients and a patient with chronic lymphocytic leukemia. The described emergence of antiviral drug resistance has led to a definite need for in vitro antiviral susceptibility testing. Phenotypic assays, although considered to be the reference methods for many years, are rarely used in clinical practice today, as they are cumbersome, labor-intensive, and expensive and have turnaround times that are far too long to be clinically relevant. Most assays measure the status of immune reconstitution by detecting the release of gamma interferon following stimula- 100. Human Cytomegalovirus n 1731 products; some assays use specific probes to detect selected mutations or use restriction endonuclease digestion of amplified products to identify alterations in the viral genome known to be associated with viral resistance to a given viral agent. Current genotypic assays, however, detect only known drugresistant mutations, and the results may be confounded by the presence of mutations that have no bearing on drug resistance. The overall complexities of phenotypic and genotypic assays make either method less than routine for most clinical laboratories. Reactivation of latent virus is also common, and other pathogens may be simultaneously present in patients with overt disease. Serial monitoring of highrisk patients also may be beneficial with respect to aiding in the decreased use of and exposure to antivirals, leading to a more cost-effective and focused use of such agents. Urine is the preferred specimen because it contains greater amounts of virus, and the virus therefore grows quickly in culture. Infants not previously tested but found to be excreting virus after 3 weeks of age may have either congenital or acquired infection. Standard serologic or virologic tests do not differentiate between these possibilities. Mengelle C, Sandres-Saune K, Pasquier C, Rostaing L, Mansuy J-M, Marty M, Da Silva I, Attal M, Massip P, Izopet J. Garrigue I, Boucher S, Couzi L, Caumont A, Dromer C, Neau-Cransac M, Tabrizi R, Schrive M-H, Fleury H, Lafon M-E. The clinical utility of whole blood versus plasma cytomegalovirus viral load assays for monitoring therapeutic response. Effect of delayed specimen processing on cytomegalovirus antigenemia test results. Dried blood spot real-time polymerase chain reaction assays to screen newborns for congenital cytomegalovirus infection. Diagnosis and monitoring of cytomegalovirus infec- cial in evaluating a pregnant woman with symptoms of viral disease. Knowing the serostatus of the donor and recipient is therefore important in determining the treatment or prophylaxis to be used and in considering the type of donor to be selected and blood products to be given. New developments in the management of cytomegalovirus infection after solid organ transplantation. Human Cytomegalovirus n tion by the quantification of viral load in dried blood spot samples. Reduced ability to culture cytomegalovirus from peripheral blood leukocytes isolated by direct erythrocyte lysis. Clinical experience with immune monitoring for cytomegalovirus in solid-organ transplant recipients. State-of-the-art monitoring of cytomegalovirus-specific cell-mediated immunity after organ transplant: a primer for the clinician. Clinical utility of viral load in management of cytomegalovirus infection after solid organ transplantation. Quantitation of human cytomegalovirus viremia by using monoclonal antibodies to different viral proteins. Comparison of quantitative cytomegalovirus antigenemia assay with culture methods and correlation with clinical disease. Monitoring antigenemia is useful in guiding treatment of severe cytomegalovirus disease after organ transplantation. Human cytomegalovirus load measurement and its applications for pre-emptive therapy in patients undergoing hematopoietic stem cell transplantation. Use of the cytomegalovirus pp65 antigenemia assay for preemptive therapy in allogeneic hematopoietic stem cell transplantation: a real-world review. Direct detection of cytomegalovirus in peripheral blood leukocytes-a review of the antigenemia assay and polymerase chain reaction. Rapid cytomegalovirus pp65 antigenemia assay by direct erythrocyte lysis and immunofluorescence staining. Standardization of the human cytomegalovirus antigenemia assay by means of in vitro-generated pp65-positive peripheral blood polymorphonuclear leukocytes. Machida U, Kami M, Fukui T, Kazuyama Y, Kinoshita M, Tanaka Y, Kanda Y, Ogawa S, Honda H, Chiba S, Mitani K, Muto Y, Osumi K, Kimura S, Hirai H.
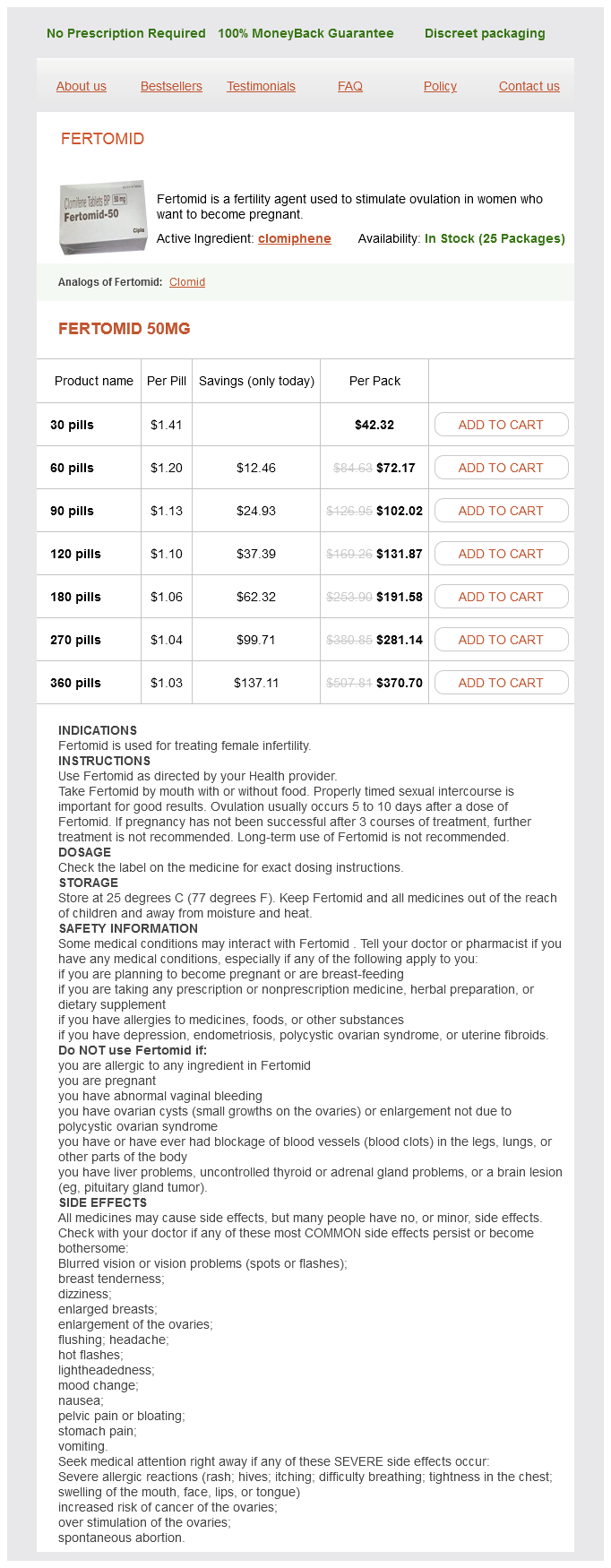
Fertomid Dosage and Price
Fertomid 50mg
- 30 pills - $42.32
- 60 pills - $72.17
- 90 pills - $102.02
- 120 pills - $131.87
- 180 pills - $191.58
- 270 pills - $281.14
- 360 pills - $370.70
Basophils have a bilobed nucleus breast cancer oncologist fertomid 50 mg buy with visa, in contrast to mast cells, which have a unilobed nucleus. More recent studies have demonstrated that despite their significant similarities, basophils and mast cells are terminally differentiated progeny of distinct bone marrow progenitors. Basophils develop from haemopoietic stem cells, mature in the bone marrow and circulate in the blood, whereas mast cells mature in the tissues. They both play significant roles in the development of a number of allergic and inflammatory disorders, as well as host defence mechanisms against parasites. Basophils arise from a common basophileosinophil progenitor cell, mature in the marrow over a period of 27 days and after their release into the circulation persist for up to 2 weeks. They are the key mediators of immediate hypersensitivity reactions such as asthma, urticaria and anaphylaxis. In addition, they have been implicated in the delayed cutaneous hypersensitivity reaction. Histamine is derived from histidine by decarboxylation and is stored as a complex with heparin or chondroitin sulfate proteoglycans. The primary protease present in mast cells, tryptase, is mainly released during the early phase of allergic response and is a marker of mast cell activation in chronic inflammatory diseases. Other neutral proteases such as carboxypeptidase B, chymase and sulfatases are also released and degrade extracellular matrix proteins. In other myeloproliferative neoplasms, elevation of basophil numbers is usually more modest. Rarely, basophils may constitute over 80% of circulating leucocytes, a condition sometimes referred to as basophilic leukaemia. Other causes of basophilia include ulcerative colitis, myxoedema, recovery from acute illness and drug allergies, although these conditions are usually associated only with modest elevations of circulatory basophils. An increased number of tissue mast cells can be seen in a number of disorders, including atopy, parasitic diseases, Hodgkin lymphoma and other lymphoproliferative disorders, certain neoplasms and rheumatoid arthritis. Several conditions, ranging from isolated cutaneous mastocytomas to mast cell leukaemia, are associated with mast cell proliferation. The more common cutaneous mastocytosis or urticaria pigmentosa typically presents with multiple, small, round, reddishbrown maculopapular lesions that, when subjected to minimal trauma, lead to intense pruritus. In some patients, this disease progresses to the systemic variety, with involvement of bone marrow, spleen, liver and the gastrointestinal tract. Systemic mastocytosis can also occur without prior or concurrent cutaneous disease, and in association with haematological disorders, including leukaemias and lymphomas. Organ dysfunction may be secondary to the release of biochemical mediators by mast cells, such as peptic ulcer disease secondary to histamine release. Mast cell leukaemia, a rare condition, presents with circulating mast cells of abnormal morphology (accounting for up to 95% of circulating nucleated cells), peptic ulcer disease, constitutional symptoms, anaemia and hepatosplenomegaly. Management of patients within all categories of mastocytosis includes careful counselling of patients and care providers, avoidance of factors triggering acute mediator release, treatment of acute and chronic mast cell mediator release, an attempt to treat organ infiltration by mast cells, and treatment of any associated non-mast-cell haematological disorder. With increased availability of small-molecular-weight inhibitors of intracellular signalling pathways, targeting of the constitutively active mutated c-kit has attracted more attention. The more frequent mutation occurs in the catalytic pocket coding region, with substitutions at codon 816, and the other in the intracellular juxtamembrane coding region. Therefore, kinase inhibitors that block mutated c-kit activity are being evaluated as therapeutic agents in systemic mastocytosis. Macrophages stimulated by macrophage colony-stimulating factor can also clear apoptotic cells by macropinocytosis. Following their release into the circulation, monocytes rapidly partition between the marginating and circulating pools. The circulating monocytes have a highly convoluted surface and a lobulated nucleus. They can be further characterized by non-specific esterase staining and contain a folded, multilobulated nucleus. After migration into tissues, they become larger and acquire the characteristics of tissue macrophages. Monocytes contain lysosomal hydrolases and the intracellular enzymes elastase and cathepsin. Kupffer cells, phagocytic cells residing within the lumen of hepatic sinusoids, represent up to 90% of fixed tissue macrophages and are the first phagocytes to encounter bacteria originating from the colon. Kupffer cells are also implicated in the removal of neutrophils after the clearance of an organism, downmodulating the inflammatory response and abrogating the tissue destruction sometimes seen in overwhelming sepsis. They stimulate monocyte/macrophage proliferation, increase adhesion receptor expression and stimulate the production of proteolytic agents responsible for pathogen clearance. Inflammatory conditions Infections Tuberculosis Bacterial endocarditis Fever of unknown origin Syphilis Other Systemic lupus erythematosus Rheumatoid arthritis Temporal arteritis Polyarteritis Ulcerative colitis Sarcoidosis Myositis Malignant disorders Acute myeloid leukaemia Hodgkin lymphoma Non-Hodgkin lymphomas Histiocytoses Carcinomas Myelodysplastic syndrome Miscellaneous Cyclic neutropenia Chronic idiopathic neutropenia Kostmann syndrome Post splenectomy monocytes has been reported with endotoxaemia, corticosteroid administration and hairy cell leukaemia. Histiocytic disorders Dendritic cells originate in the bone marrow and share a common progenitor with macrophages. Precursors of dendritic cells are released from the bone marrow and enter tissues in which they differentiate into functional antigen-presenting dendritic cells. Tissue-based dendritic cells comprising the dendritic cell system lack phagocytic capacity or Fc receptors and are predominantly antigen-presenting cells. The dendritic Langerhans cells are found in virtually all tissues except the brain and are the major immunological cellular components of the skin and mucosa. Their racquet-shaped ultrastructural inclusions (Birbeck bodies) distinguish them from other tissue cells. They interact with and process antigen, then migrate to lymphoid organs where, through interaction with T cells, they generate cellular and humoral immune responses.