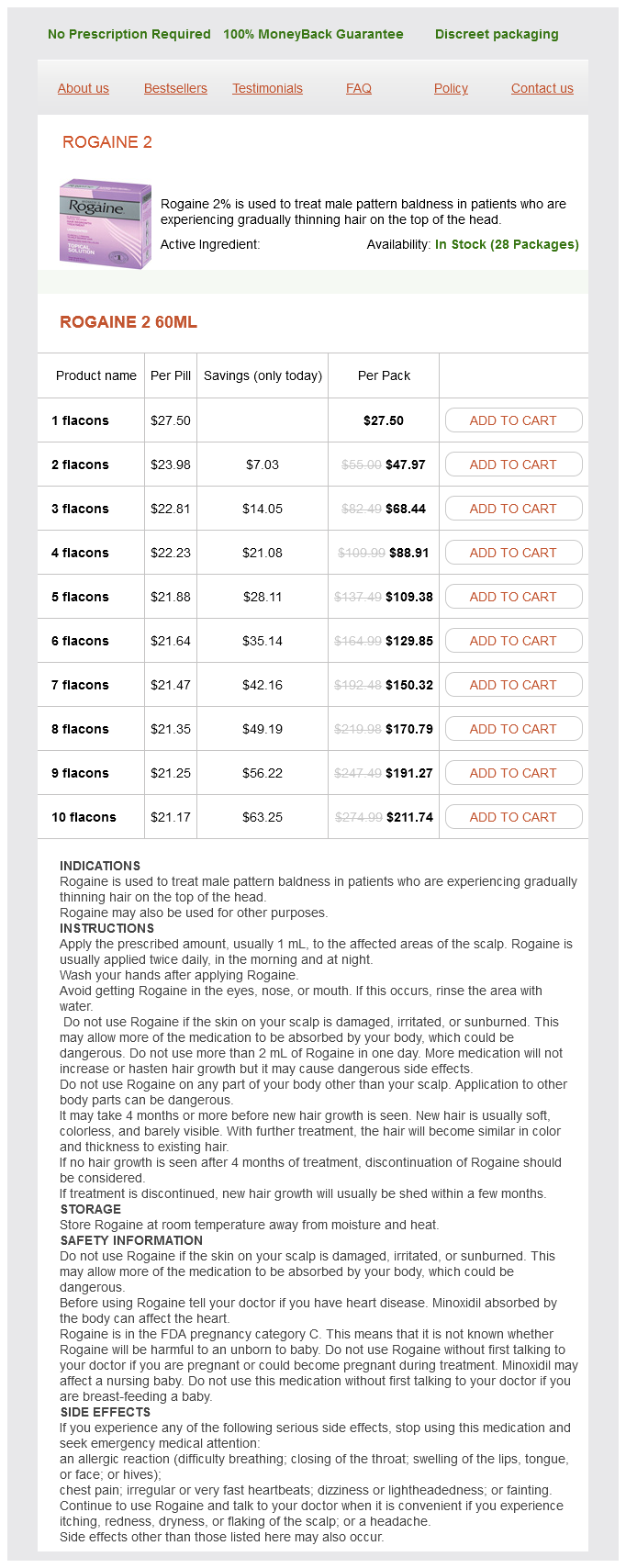
Rogaine 2
General Information about Rogaine 2
It is a topical resolution that incorporates the active ingredient minoxidil, which is the one FDA-approved ingredient for hair regrowth.
Male sample baldness, also referred to as androgenetic alopecia, is a standard situation that affects millions of males worldwide. It is characterised by a receding hairline and gradual thinning of hair on the highest of the head, resulting in a definite 'M' shape. While it's a pure situation associated with getting older, many males nonetheless feel self-conscious about their hair loss and seek ways to revive their full head of hair. This is where Rogaine 2% is obtainable in.
Rogaine 2% is a topical solution that is applied directly to the scalp to stimulate hair progress. It works by dilating blood vessels and rising blood move to the hair follicles, selling hair development and stopping additional hair loss. The lively ingredient in Rogaine 2% is minoxidil, a vasodilator that was originally developed as an oral medication to treat hypertension. However, throughout medical trials, it was found to have a aspect effect of increased hair growth, leading to its discovery as a remedy for male pattern baldness.
In conclusion, Rogaine 2% is a well-liked and efficient treatment for male pattern baldness. Its energetic ingredient, minoxidil, has been confirmed to promote hair progress and forestall additional hair loss. With its simple utility and potential for noticeable leads to as little as eight weeks, it's no wonder that Rogaine 2% continues to be a go-to choice for men looking to combat hair loss. However, as with every medicine, it is always greatest to seek the advice of with a healthcare professional earlier than starting remedy to make sure it is the proper possibility for you.
One of the primary benefits of using Rogaine 2% is its convenience. It is a non-invasive therapy that can be simply utilized at home without the necessity for a prescription. Its packaging features a dropper for precise application and a foam applicator for a quick and mess-free process. Just a couple of drops utilized to the affected area twice a day is adequate to see noticeable ends in as little as eight weeks.
It is necessary to notice that Rogaine 2% isn't a remedy for male pattern baldness, but rather a remedy that can assist slow down or even reverse hair loss. Results might differ from individual to individual, and the quantity and price of hair regrowth cannot be predicted. It can be important to manage expectations and understand that Rogaine 2% can't bring again a full head of hair for somebody who has been completely bald for many years.
Like any treatment, Rogaine 2% may have some side effects. The most common side impact is scalp irritation, which may include redness, itchiness, and flaking. This can often be managed by reducing the frequency of use or using the foam formula instead of the liquid solution. Some users may experience minor shedding or elevated hair loss in the course of the first few weeks of use. This is a common and temporary incidence often recognized as the 'dread shed' and is an indication that the product is working to push out old, weak hair and make way for brand spanking new, more healthy strands.
Rogaine 2% works greatest for people who are in the early levels of hair loss and have a healthy scalp. It is not designed for treating receding hairlines, bald patches, or hair loss attributable to components aside from male sample baldness. It also requires constant and long-term use to see outcomes. If treatment is discontinued, the hair regrowth achieved via using Rogaine 2% may be misplaced inside a couple of months.
Because this reaction is catalyzed by an enzyme mens health 17 day abs purchase rogaine 2 in united states online, it is also appropriate to call water and carbon dioxide. The speed at which this reaction occurs is called the reaction, often expressed as molarity/second. Match each definition in the left column with the correct term from the right column (you will not use all the terms): (a) reaction that can run either direction (b) reaction that releases energy (c) ability of an enzyme to catalyze one reaction but not another (d) boost of energy needed to get a reaction started 1. Organic molecules that must be present in order for an enzyme to function are called. In an oxidation-reduction reaction, in which electrons are moved between molecules, the molecule that gains an electron is said to be, and the one that loses an electron is said to be. Transfer of an amino group from one molecule to the carbon skeleton of another molecule (to form a different amino acid) is called. In metabolism, reactions release energy and result in the breakdown of large biomolecules, and reactions require a net input of energy and result in the synthesis of large biomolecules. Metabolic regulation in which the last product of a metabolic pathway (the end product) accumulates and slows or stops reactions earlier in the pathway is called. List two carrier molecules that deliver high-energy electrons to the electron transport system. Explain why it is advantageous for a cell to store or secrete an enzyme in an inactive form. When bonds are broken during a chemical reaction, what are the three possible fates for the potential energy found in those bonds Match the metabolic processes with the letter of the biological theme that best describes the process: (a) biological energy use (b) compartmentation (c) molecular interactions 1. Glycolysis takes place in the cytosol; oxidative phosphorylation takes place in mitochondria. The electron transport system traps energy in a hydrogen ion concentration gradient. For two days, the patient received a slow drip of fluid into his veins directly from young coconuts suspended next to his bed. A salty taste meant that the child was destined to die of a mysterious disease that withered the flesh and robbed the breath. Today, a similar "sweat test" will be performed in a major hospital-this time, with state-of-the-art techniques-on Daniel Biller, an 18-month-old with a history of weight loss and respiratory problems. Water is essentially the only molecule that moves freely between cells and the extracellular fluid. Because of this free movement of water, the extracellular and intracellular compartments reach a state of osmotic equilibrium 5 osmos, push or thrust 6, in which the fluid concentrations are equal on the two sides of the cell membrane. Calcium (not shown in the figure) is more concentrated in the extracellular fluid than in the cytosol, although many cells store Ca2+ inside organelles such as the endoplasmic reticulum and mitochondria. Proteins and other large anions are concentrated in the plasma but cannot cross the leaky exchange epithelium of blood vessels [p. On the other hand, smaller molecules and ions such as Na+ and Cl- are small enough to pass freely between the endothelial cells and therefore have the same concentrations in plasma and interstitial fluid. The concentration differences of chemical disequilibrium are a hallmark of a living organism, as only the continual input of energy keeps the body in this state. If solutes leak across the cell membranes dividing the intracellular and extracellular compartments, energy is required to return them to the compartment they left. When cells die and cannot use energy, they obey the second law of thermodynamics [p. Many body solutes mentioned so far are ions, and for this reason we must also consider the distribution of electrical charge between the intracellular and extracellular compartments. The body as a whole is electrically neutral, but a few extra negative ions are found in the intracellular fluid, while their matching positive ions are located in the extracellular fluid. As a result, the inside of cells is slightly negative relative to the extracellular fluid. The intracellular and extracellular compartments of the body are in osmotic equilibrium, but in chemical and electrical disequilibrium. Furthermore, osmotic equilibrium and the two disequilibria are dynamic steady states. In the remainder of this chapter, we discuss these three steady states, and the role transport mechanisms and the selective permeability of cell membranes play in maintaining these states. Substances moving between the plasma and interstitial fluid must cross the leaky exchange epithelium of the capillary wall. Use your answers from the two questions above to calculate the percentage of total body water in the plasma and interstitial fluid. In clinical situations, we monitor homeostasis of various substances such as ions, blood gases, and organic solutes by taking a blood sample and analyzing its plasma. In this section, we examine the relationship between solute movement and water movement across cell membranes. Infants have relatively more water than adults, and water content decreases as people grow older than 60. In clinical practice, it is necessary to allow for the variability of body water content when prescribing drugs. Because women and older people have less body water, they will have a higher concentration of a drug in the plasma than will young men if all are given an equal dose per kilogram of body mass. The remaining third (33%) is split between the interstitial fluid (which contains about 75% of the extracellular water) and the plasma (which contains about 25% of the extracellular water). The Body Is in Osmotic Equilibrium Water is able to move freely between cells and the extracellular fluid and distributes itself until water concentrations are equal throughout the body-in other words, until the body is in a state of osmotic equilibrium. The movement of water across a membrane in response to a solute concentration gradient is called osmosis.
The relaxed diaphragm has a dome shape that protrudes upward into the thoracic cavity man health after 50 generic 60 ml rogaine 2. This representation is not to scale; the diameter of an alveolus is actually about 600 times larger than the space between the alveolus and a pulmonary capillary. At rest, the costal parts are active, but as respiratory demands increase, additional motor units of the costal diaphragm and then the crus are recruited. The abdominal wall, if relaxed, moves outward during inspiration as the descending diaphragm pushes the abdominal contents downward and forward. Fifty to 75 percent of the enlargement of the thoracic cavity during quiet inspiration is accomplished by contraction of the diaphragm. Two sets of intercostal muscles lie between the ribs (inter means "between"; costa means "rib"). Contraction of these muscles, whose fibres run downward and forward between adjacent ribs, enlarges the thoracic cavity in both the lateral (side-to-side) and anteroposterior (front-to-back) dimensions. When the external intercostals contract, they elevate the ribs, moving the sternum upward and outward. Many respiratory physiologists think the main result of activation of the external intercostal muscles is to stabilize the chest wall, preventing it from being sucked in during inspiration. This increases the efficiency of the diaphragm because its contraction is used to inflate the lung rather than being wasted on moving the chest wall. Muscles of inspiration Sternocleidomastoid Scalenes Muscles of expiration Why It Matters External intercostals Parasternal intercostals Diaphragm Internal intercostals Pleurisy, an inflammation of the pleural membrane, is accompanied by painful breathing, because each inflation and deflation of the lungs cause a "friction rub" between the pleural and visceral membranes. External abdominal oblique lubricates the surfaces of the two membranes as they slide past each other during breathing. In humans, these are inactive at rest in healthy individuals but are recruited when ventilatory demands increase, as during exercise. They are also recruited during such protective reflexes as coughing, sneezing, and vomiting. Because these reflexes are so important, the expiratory muscles can generate much higher pressures than the inspiratory muscles. The internal intercostals are innervated by branches of the same thoracic nerves that project to the external intercostals. The abdominal muscles are innervated by nerves originating from spinal cord segments T7 through L1. The pressure gradient is used to overcome the elastance (stiffness) of the respiratory system, the resistance to airflow, and the inertia of the system. For flow to occur between the airway opening (nose, mouth) and alveoli, pressure in the alveoli must be less than pressure at the mouth. For expiration to occur, pressure in the alveoli must be greater than the pressure at the mouth. To understand how our body changes alveolar pressure requires an introduction to the mechanics of the respiratory system. List the three factors opposing changes in volume of the respiratory system, and indicate their relative importance. Schematic representation of the relationship of the pleural space to the lungs and thorax. The inside membrane of the pleural space, the visceral pleura, closely adheres to the surface of the lung and then reflects back on itself to form the other membrane, the parietal pleura, which lines the interior surface of the thoracic wall. The size of the pleural space between these two layers is grossly exaggerated here for the purpose of visualization. Due to electronic rights, some third party content may be suppressed from the eBook and/or eChapter(s). Pleural pressure (PpI) is the pressure outside the lungs but still within the thoracic cavity; that is, the pressure within the pleural space. It is subatmospheric (negative) because of the mechanical properties of the lungs and the chest wall; the former wants to collapse and the latter expand, creating the negative pressure. In this depiction, flow is zero, and all pressures are in cmH2O, relative to atmospheric pressure set to 0 cmH2O. The pressure gradient across a structure is called the transmural pressure (trans is Latin for "across"; mural is the adjective of murus, Latin for "wall"). A transmural pressure difference is always calculated as the inside pressure minus the outside pressure. When referring to the lung, the transmural pressure is called the transpulmonary pressure (Ptp). As lung volume increases, its recoil pressure increases from ~ 0cmH 2 O at residual volume to about 30cmH 2 O at total lung capacity (p. The lung therefore behaves like a balloon or a bubble, always exerting a positive (deflating) pressure. Below 65 percent, however, it is like a compressed spring, exerting negative (inflating) pressures. The solid line labelled Prs shows the pressure volume relationship of the respiratory system. Here, the pressures represent the sums of the pressures of the lung and chest wall at any given volume. At approximately 40 percent of the vital capacity, however, the net pressure exerted by the respiratory system is 0cmH 2 O because, at this volume, the deflating pressure of the lung (about 15cmH 2 O) is equal and opposite to the inflating pressure (25cmH 2 O) of the chest wall. For example, a pressure of 5 cmH2O, which can easily cause a high flow, equals ~0. In addition, pressures related to convective flow are expressed relative to atmospheric pressure.
Rogaine 2 Dosage and Price
Rogaine 2 60ml
- 1 flacons - $27.50
- 2 flacons - $47.97
- 3 flacons - $68.44
- 4 flacons - $88.91
- 5 flacons - $109.38
- 6 flacons - $129.85
- 7 flacons - $150.32
- 8 flacons - $170.79
- 9 flacons - $191.27
- 10 flacons - $211.74
For years prostate location in body rogaine 2 60 ml low cost, researchers knew of a short-lived signal molecule produced by the endothelial cells lining blood vessels. This molecule diffuses from the endothelium into adjacent smooth muscle cells, causing the muscle to relax and dilate the blood vessel. Aequorin, a protein complex isolated from jellyfish such as the Chrysaora fuscescens shown here, is one of the molecules that scientists use to monitor the presence of calcium ions. When aequorin combines with calcium, it releases light that can be measured by electronic detection systems. Since the first use of aequorin in 1967, researchers have been designing increasingly sophisticated indicators that allow them to follow calcium signals in cells. And since the 1860s, physicians have used nitroglycerin to relieve angina, heart pain that results from constricted blood vessels. Even today, heart patients carry little nitroglycerin tablets to slide under their tongues when angina strikes. Still, it took years of work to isolate nitric oxide, the short-lived gas that is the biologically active molecule derived from nitroglycerin. Garlic is a major dietary source of the sulfur-containing precursors, which may explain studies suggesting that eating garlic has protective effects on the heart. Arachidonic acid itself may act directly as a second messenger, altering ion channel activity and intracellular enzymes. It may also be converted into one of several classes of eicosanoid paracrine signals. These lipid-soluble molecules can diffuse out of the cell and combine with G protein-coupled receptors on neighboring cells to exert their action. There are two major groups of arachidonic acid-derived paracrine molecules to be aware of: 1. Leukotrienes are molecules produced by the action of the enzyme lipoxygenase on arachidonic acid leuko-, white + triene, a molecule with three double bonds between carbon atoms. They play a significant role in asthma, a lung condition in which the smooth muscle of the airways constricts, making it difficult to breathe, and in the severe allergic reaction known as anaphylaxis. For this reason, pharmaceutical companies have developed drugs to block leukotriene synthesis or action. These eicosanoids act on many tissues of the body, including smooth muscle in various organs, platelets, kidney, and bone. However, Some Lipids Are Important Paracrine Signals One of the interesting developments from sequencing the human genome and using genes to find proteins has been the identification of orphan receptors, receptors that have no known ligand. Scientists are trying to work backward through signal pathways to find the ligands that bind to these orphan receptors. It was from this type of research that investigators recognized the importance and universality of eicosanoids, lipid-derived paracrine signals that play important roles in many physiological processes. Eicosanoid signal molecules are derived from arachidonic acid, a 20-carbon fatty acid and act on their target cells using G protein-coupled receptors. Arachidonic acid-derived molecules such as eicosanoids are not the only known lipid signal molecules. Sphingolipids also act as extracellular signals to help regulate inflammation, cell adhesion and migration, and cell growth and death. Like the eicosanoids, sphingolipids combine with G protein-coupled receptors in the membranes of their target cells. The a isoform has a higher binding affinity for norepinephrine, and the b2 isoform has a higher affinity for epinephrine. Agonists and Antagonists When a ligand combines with a receptor, one of two events follows. A competing ligand that binds and elicits a response is known as an agonist of the primary ligand. Competing ligands that bind and block receptor activity are called antagonists of the primary ligand. One example is the family of modified estrogens (female sex hormones) in birth control pills. These drugs are agonists of naturally occurring estrogens but have chemical groups added to protect them from breakdown and extend their active life. Use what you have learned about leukotrienes, signal molecules, and signal transduction to predict what these drugs are acting to have those effects. One Ligand May Have Multiple Receptors To complicate matters, different cells may respond differently to a single kind of signal molecule. For most signal molecules, the target cell response depends on its receptor or its associated intracellular pathways, not on the ligand. For many years physiologists were unable to explain the observation that a single signal molecule could have different effects in different tissues. For example, the neurohormone epinephrine dilates blood vessels in skeletal muscle but constricts blood vessels in the intestine. The answer became clear when scientists discovered that epinephrine was binding to different adrenergic receptor isoforms in the two tissues. Receptors Exhibit Saturation, Specificity, and Competition Because receptors are proteins, receptor-ligand binding exhibits the general protein-binding characteristics of specificity, competition, and saturation (discussed in [Chapter 2, p. Receptors, like enzymes and transporters, also come as families of related isoforms [p. Specificity and Competition: Multiple Ligands for One Receptor Receptors have binding sites for their ligands, just as enzymes and transporters do.